|
Acid Rock Drainage and Metal Leaching at the Pebble Prospect Prepared by Dr. Kendra Zamzow, Center for Science in Public Participation , www.csp2.org Background Why is “acid drainage” a concern at mines? What causes it? If there isn’t acid, do metals still cause problems for fish? Is “acid mine drainage” a concern at the Pebble prospect? Acid drainage and metal leaching occur because the metals we want from mines commonly exist as complex chemical compounds in the rock. The entire mineral makeup of the rock, not just the copper or gold, determines how excavating and storing rock affects the environment. Three processes can lead to contamination. First, rain and snow falling on crushed or broken rock can turn the water acidic (low pH) or alkaline (high pH). Second, rain or snow on rock may leach metal salts (readily dissolved compounds) into water. Third, processing chemicals can leak or spill. What is Acid Rock Drainage (ARD)? Iron is one of the most common elements on earth, and it is the iron sulfide that often occurs with gold and other valuable minerals that is the main source of acid. Rain, snowmelt, or water moving over iron sulfide forms sulfuric acid. The acid dissolves metals in the rock like copper, zinc, nickel, and lead. Acid and metals are washed downstream into clean watersheds where aquatic plants and animals are exposed. This process is called “acid rock drainage” or, because it occurs at many mining sites, “acid mine drainage”. It can occur in tunnels, open pits, waste rock piles, and mill waste (tailings) (Figure 1).
Rust is oxidized iron. Dissolved oxidized iron gives acid drainage its distinctive red color. These processes occur naturally, but are more extreme when rock is crushed and more rock surface is exposed. While sulfide makes acid, carbonate in rock buffers acid. The ratio of sulfides to neutralizing rock like carbonate influences the overall acidity of mine drainage. Environmental Effects of ARD Acid mine drainage may contain copper, zinc, cadmium and other minerals to which salmon are very sensitive. As little as a 2-8 parts per billion increases in copper above natural stream levels damages the ability of salmon to smell and it becomes harder to avoid predators, find mates, and return to spawning grounds. When acid drainage enters clean streams, the acid is diluted, but “yellowboy” forms as red dissolved iron becomes solid. Yellowboy is a classic orange color (Figure 2) and acts like cement, smothering species that live on stream bottoms.
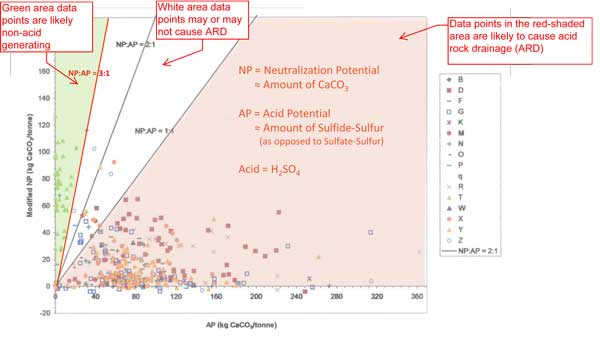
Dissolved aluminum also becomes solid in natural waters, forming mucus-like streamers that clog fish gills and cause fish to suffocate. What is Metal Leaching? Metals and metal-like elements don’t need acid to dissolve – they can dissolve at neutral or alkaline pH. This is called “metal leaching”. Alkaline pH can come about in two ways: if the rock contains a lot of carbonate, or if ore processing requires the pH of process water to be very high. For instance, when cyanide is used to extract gold, the pH must be kept high to avoid forming cyanide gas that can kill people. Alkaline water causes arsenic, cadmium and selenium to dissolve. These toxins can reduce growth, cause physical deformities and kill fish. Can Acid Drainage Be Predicted? As little as 0.2 percent sulfide in rocks may trigger acid drainage, depending on site conditions. One 2006 scientific study of 25 major US metal mines found that 76% of them exceeded water quality standards. Where there was a likelihood of acid drainage or metal leaching and the mine was near water, the water became contaminated in almost every case. Perpetual Pollution Acid drainage is irreversible. Placing sulfide rock underwater or burying it can slow acid formation by removing oxygen, but it won’t stop completely if certain forms of iron are present. Since it cannot be stopped, the contaminated water must be treated in perpetuity, for hundreds or thousands of years. Mining companies post bonds to pay for water treatment, but regulators often underestimate the cost, leaving taxpayers to pay. For example, when Montana and the US Environmental Protection Agency (EPA) requested Pegasus Gold to clean up water pollution in 1998, Pegasus filed for bankruptcy and left Montana with millions of dollars in water treatment costs. Acid Mine Drainage and the Pebble Prospect Preliminary geochemical data indicates significant acid mine drainage potential at Pebble. If water treatment is required, expensive lime treatment may be necessary, possibly in perpetuity. Lime products form sludge that will require on site disposal. Treated discharge has low metals but high total dissolved solids (tds). Authorizing a mine where it is known that water treatment in perpetuity will be required poses significant long term financial and/or environmental risks to the public. Acid generating potential (AP) of 399 samples from the Pebble West deposit, 1988-20034. Explanations by D. Chambers. (click on figure for a larger view) This summary was written by Dr. Kendra Zamzow of CSP2, http://www.csp2.org. Further Reading and References Acid Mine Drainage and Effects on Fish Health and Ecology: A Review (pdf file 193 kb) |